43 medication labels must include
Tobacco packaging warning messages - Wikipedia As of 30 January 2013, all cigarette packages must include graphical warning labels that show the detrimental effects on the health of long-term smokers. Translation of words in box: Smoking is very bad for health. It can cause heart disease, gangrene, lung cancer, reduced lung function, chronic bronchitis, stroke, throat and mouth cancer, and ... Blacking Out Names in Medical Records: Privacy and HIPAA ... The holder of that information must supply the documents to the person within 30 days. It is important to note that while people have the right to obtain their medical records, it is not unlawful for their healthcare provider to charge them a fee for delivering a copy of the records.
Drug Calculations Quiz Questions With Answers - ProProfs The medications scheduled for your patient include Keflex 1.5 grams in 50 ml of a 5% Dextrose solution. According to the pharmacy, this preparation should be administered in 30 minutes. You would set your IV pump at __________mL/hour. A. 100 mL/hr B. 110 mL/hr C. 115 mL/hr D. 120 mL/hr 8. The physician reduces an IV to 30 ml/hour.
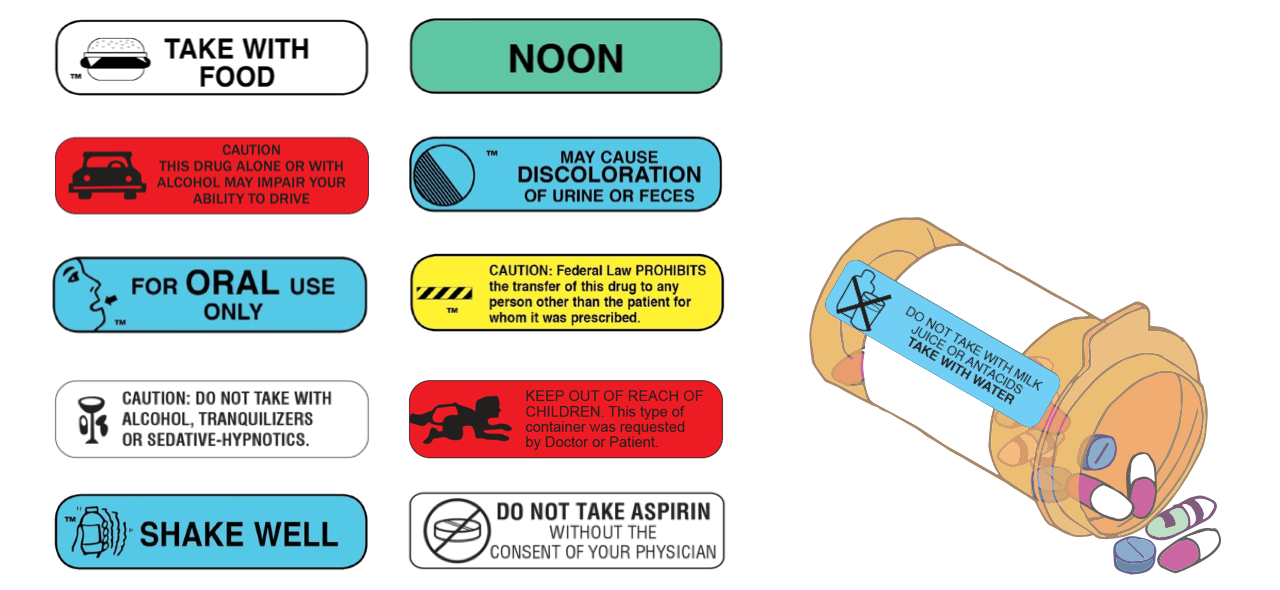
Medication labels must include
Tetracycline (Class) (Oral Route, Parenteral ... - Mayo Clinic For oral dosage form (capsules): For bacterial or protozoal infections: Adults and teenagers—250 to 500 milligrams (mg) every six hours. Children older than 8 years of age—Dose is based on body weight. The usual dose is 6.25 to 12.5 mg per kilogram (kg) (2.8 to 5.7 mg per pound) of body weight every six hours. Medicare Prescription Drug Appeals & Grievances | CMS December 2019: The Parts C and D Enrollee Grievance, Organization/Coverage Determinations and Appeals Guidance has been updated to include recent regulatory changes and will be effective January 1, 2020. Questions related to the guidance or appeals policy may be submitted to the Division of Appeals Policy at . Federal Policy Guidance | Medicaid.gov SHARE THIS FEDERAL POLICY GUIDANCE RECORD. Promoting Continuity of Coverage and Distributing Eligibility and Enrollment Workload in Medicaid, the Children's Health Insurance Program (CHIP), and Basic Health Program (BHP) Upon Conclusion of the COVID-19 Public Health Emergency. Date: 03/03/2022.
Medication labels must include. services.gileadhiv.com › content › pdfPATIENT ENROLLMENT FORMTO BE COMPLETED BY THE PATIENT OR ... The patient (or the patient’s representative) must sign and date this section. TO BE COMPLETED BY THE PRESCRIBER: ⊲ SECTION 7 (REQUIRED) Complete all fields with the prescriber’s information.* ⊲ SECTION 8 (REQUIRED) A healthcare provider must provide the patient’s diagnosis and medical information. ⊲ SECTION 9 (REQUIRED) Manufacturer Copay Cards: Everything You Need to Know - GoodRx As prescription medication prices continue to rise, it's no surprise that patients are looking for ways to save.When insurance isn't enough, many people turn to manufacturer copay cards to help offset some of the costs.. These savings programs, which come directly from medication manufacturers, can help patients who are struggling to afford expensive brand-name medications. Emergency Use Authorization | FDA - U.S. Food and Drug ... Detailed Information for all COVID-19 EUAs, including authorizations and fact sheets Vaccines Drugs and Non-Vaccine Biological Products COVID-19 EUAs for Medical Devices, including: Blood... National Drug Code Directory - FDA A labeler may be a manufacturer, including a repackager or relabeler, or the entity named on the product label. The NDC Directory contains product listing data submitted for all finished drugs...
How To Display Your Nursing Credentials | Examples Therefore, you must include if you're an RN or an LPN immediately following your degree. Margaret Miranda is a Registered Nurse therefore the letters RN follow here educational degree (MSN). Show Me Nursing Programs. 3. Specialty. If you've gone on to earn any additional advanced nursing specialization, you'll want to list that ... Local Coverage Determinations | CMS What is an LCD? Local coverage determinations (LCDS) are defined in Section 1869(f)(2)(B) of the Social Security Act (the Act). This section states: "For purposes of this section, the term 'local coverage determination' means a determination by a fiscal intermediary or a carrier under part A or part B, as applicable, respecting whether or not a particular item or service is covered on an ... Self administered medicines in care homes | CQC Public Website Your process for self administration of medicines (including controlled drugs) should include: an individual risk assessment obtaining or ordering medicines storing medicines keeping records supporting people to take their medicines if necessary monitoring adherence disposing of unwanted medicines Individual risk assessment Citalopram: 7 things you should know - Drugs.com Drugs.com provides accurate and independent information on more than 24,000 prescription drugs, over-the-counter medicines and natural products. This material is provided for educational purposes only and is not intended for medical advice, diagnosis or treatment. Data sources include IBM Watson Micromedex (updated 3 May 2022), Cerner Multum™ (updated 28 Apr 2022), ASHP (updated 11 Apr 2022 ...
info.ncdhhs.gov › dhsr › aclsMedication Study Guide - NC DHHS requirements for medication administration in adult care homes in North Carolina became effective January 1, 2000. One of the requirements is that any unlicensed person administering medications or supervising the administration of medications must pass a test given by the state of North Carolina. Information such as blog.cureatr.com › the-importance-of-medicationThe Importance of Medication Administration: 5 Ways to Improve Mar 13, 2019 · Misreading medication names that look similar is a common mistake, particularly in hospitals, and particularly when orders are communicated verbally. To reduce the risk of these errors, use strategies such as: Use “Tall Man Lettering" - which helps distinguish similar drug names; Add warning labels that alert staff to LASA drugs 2022 Nursing Diagnosis Guide | List, Examples & Types Examples of this type of nursing diagnosis include: Risk for imbalanced fluid volume Risk for ineffective childbearing process Risk for impaired oral mucous membrane integrity This type of diagnosis often requires clinical reasoning and nursing judgement. 3. Health promotion diagnosis Reporting medicine related incidents | CQC Public Website This must include all the facts, to the best of your knowledge, at the time. Tell them in person what further enquiries you will need to make. Offer an apology in person. Follow this by giving the same information in writing. Give an update on the enquiries. Keep a written record of all communication with the relevant person. Notifications
Nursing Care Plan (NCP): Ultimate Guide and ... - Nurseslabs Writing the best nursing care plan requires a step-by-step approach to complete the parts needed for a care plan correctly. This tutorial has the ultimate database and list of nursing care plans (NCP) and NANDA nursing diagnosis samples for our student nurses and professional nurses to use — all for free! A care plan's components, examples, objectives, and purposes are included with a ...
What Is a Unit Dose? (with pictures) - Info Bloom Aside from the barcode, each label contains the brand name of the medication and the unit dose measurement. In some cases, the patient's name is also printed on the label. Most medical facilities require the use of a double-check system — meaning the medication is checked by two people — for high-risk drugs.
WHMIS 1988 - Labelling Requirements : OSH Answers Are there different types of labels? Yes. A WHMIS label can be a mark, sign, stamp, sticker, seal , ticket, tag or wrapper. It can be attached, imprinted, stencilled or embossed on the controlled product or its container. However, there are two different types that are used most often: the supplier label and the workplace label.
Antihypertensive Drugs Nursing Pharmacology Study Guide These are the important things the nurse should include in conducting assessment, history taking, and examination: Assess for the mentioned contraindications to this drug (e.g. renal impairment, hyponatremia, hypovolemia, etc.) to prevent potential adverse effects.
WHMIS 2015 - General : OSH Answers true copies of labels and safety data sheets in both official languages, and documents detailing the required sales and purchasing information. Inspectors may also examine safety data sheets and labels to verify compliance with the requirements of the HPA and/or the HPR. What does it mean when I see a generic chemical identity listed on a SDS?
› resources › safety-enhancements-everySafety Enhancements Every Hospital Must Consider in Wake of ... Jan 17, 2019 · Place auxiliary labels on all storage locations and/or ADC pockets/ drawers/lids that contain neuromuscular blockers that clearly warn that respiratory paralysis will occur, and ventilation is required (e.g., “WARNING: CAUSES RESPIRATORY ARREST—PATIENT MUST BE VENTILATED”). The warning should be visible when ADC pockets/drawers/lids are open.
Document Vaccination - Recordkeeping Handouts for ... View All Materials. Dates of current Vaccine Information Statements (VISs) Print and cut out up to four charts (4" x 5.5") of current VIS dates for posting around the clinic and work place [#P2029] Declination of influenza vaccination. Form for healthcare worker signature and date, lists important reasons for annual influenza vaccination and ...
Guide to Nurse Practitioner Prescribing Laws The Nurse Practitioner controlled substance prescribing medications must include the DEA registration number. For Schedule II and II drugs, the Nurse Practitioner is authorized to prescribe a 30-day dosage supply. With the ongoing opioid crisis, the focus on prescriptive authority and ethical ramifications have taken center stage.




Post a Comment for "43 medication labels must include"